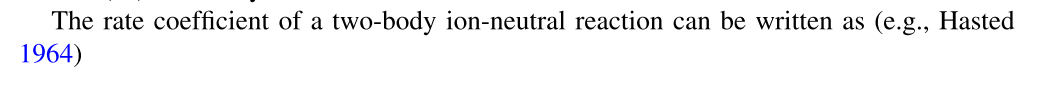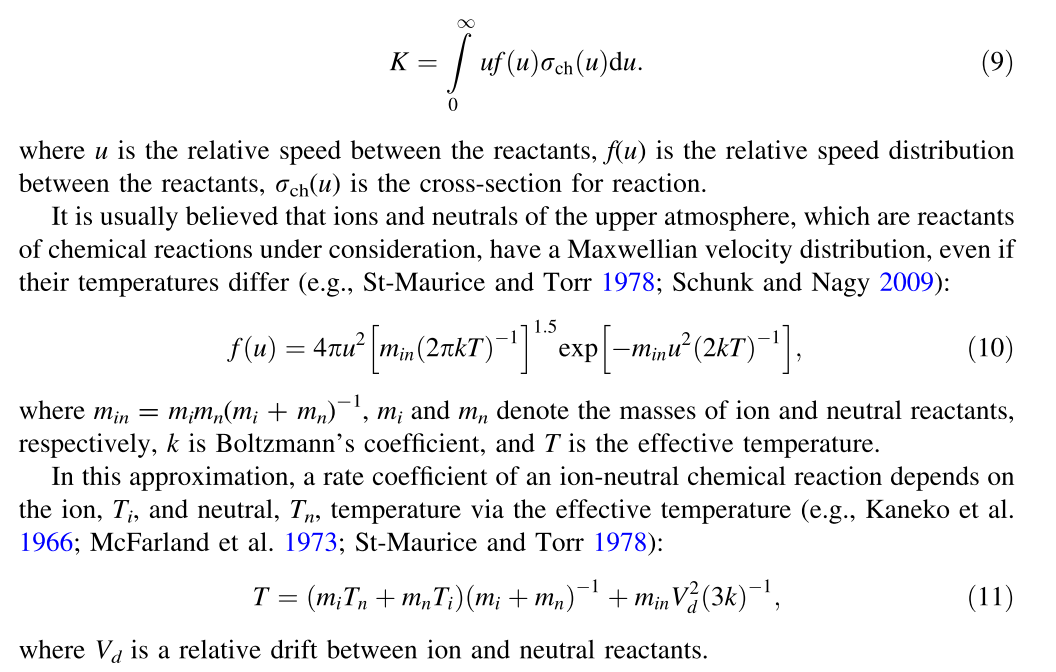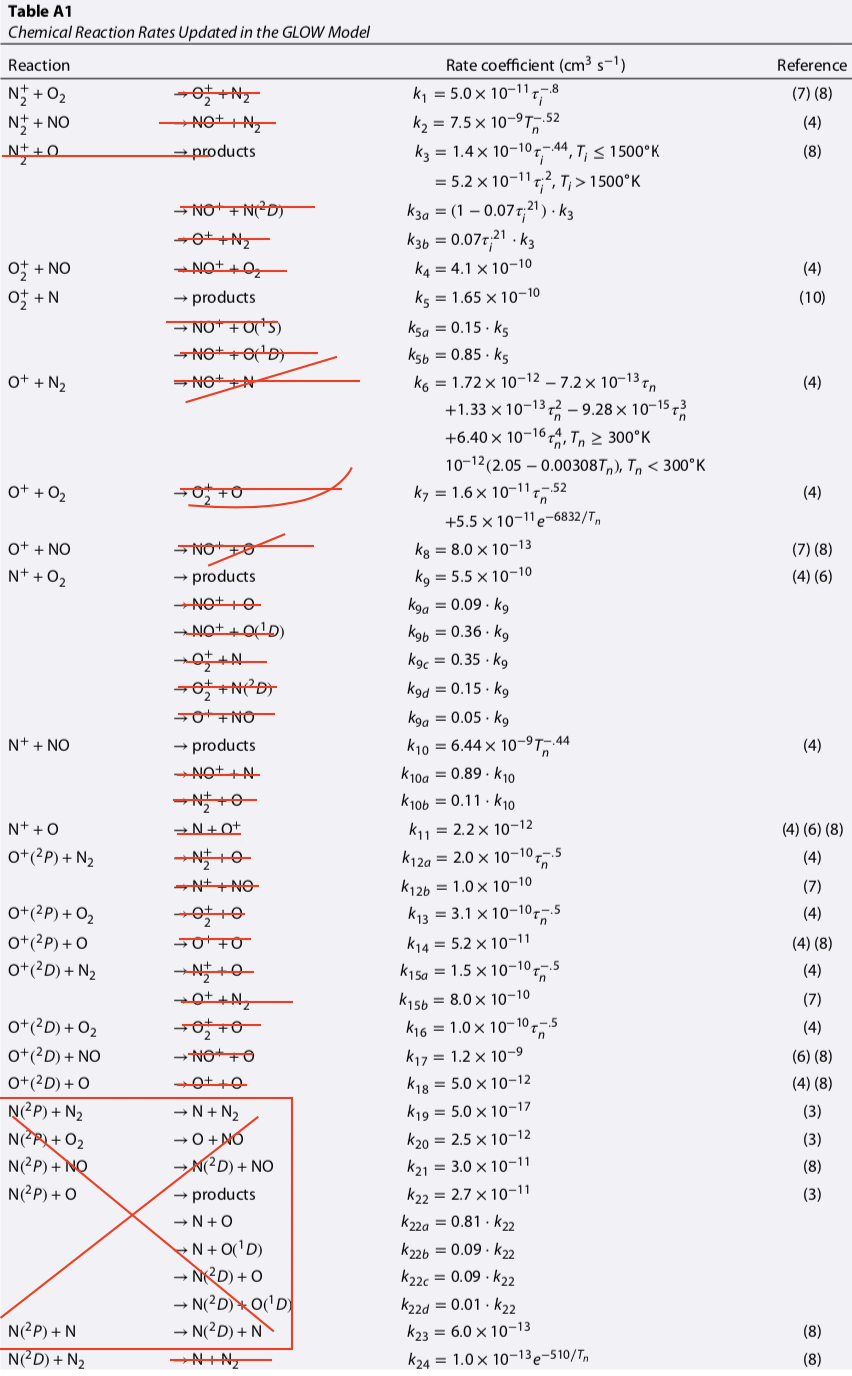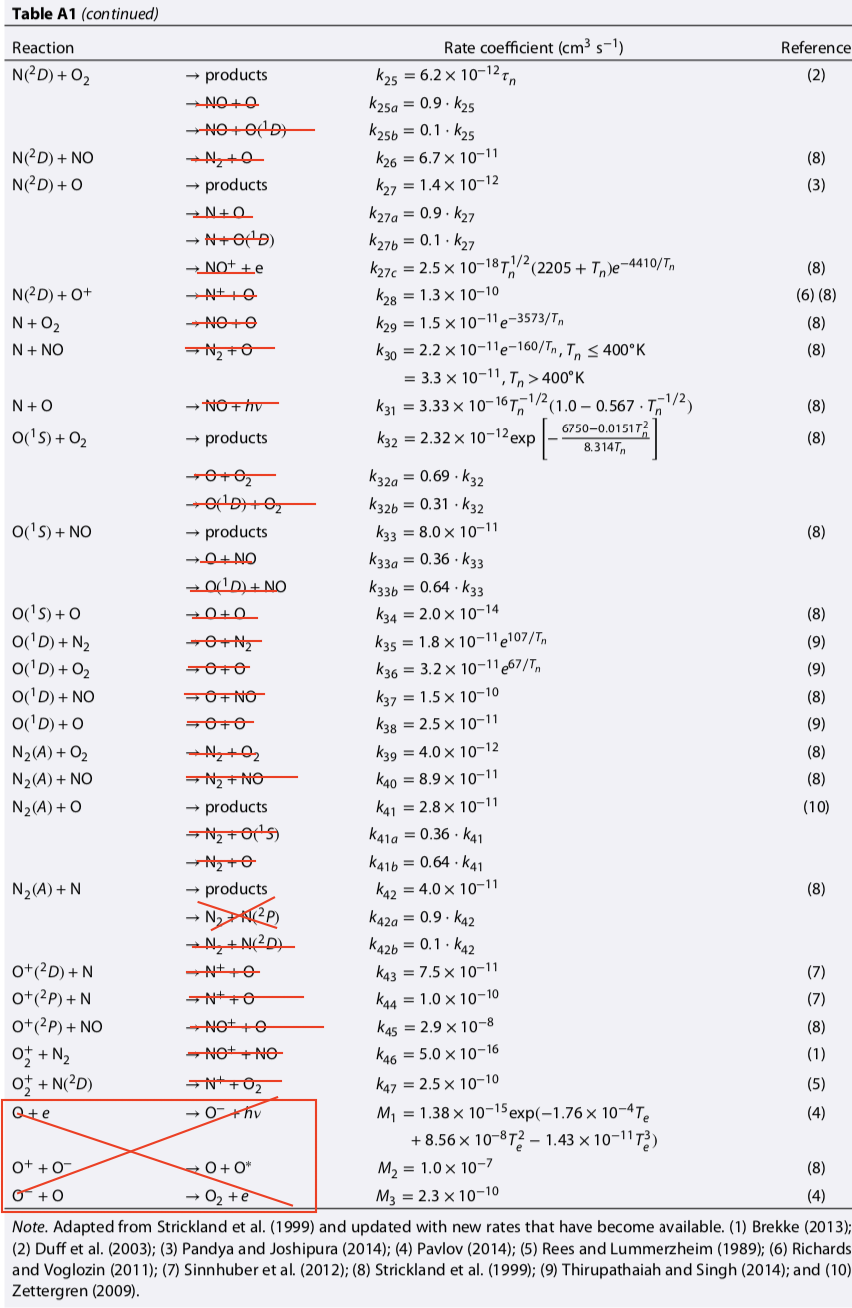
# | Reaction | Rate [cm^-3 s^-1] | Prox Src | Ult Src | Notes |
1 | N2+ + O → NO+ + N(2D) | if Ti<1500: (1-0.07*xi**0.21) * 1.4e-10*xi**-0.44 if Ti>=1500: (1-0.07*xi**0.21) * 5.2e-11*xi**0.2 | G18 | B92 has 1.4e-10*(T/300)**0.44 (same form but T instead of Tf) S88 has 1.4e-10*(Tf/300)**0.44 and lists N2D yield at 1.0 | |
39 | N2+ + O → O+ + N2 | if Ti<1500: 0.07*xi**0.21 * 1.4e-10*xi**-0.44 if Ti>1500: 0.07*xi**0.21 * 5.2e-11*xi**0.2 | G18 | Not in S88 nor B92, unless I missed it. Note this is a different branch than the above -- this yields more as Ti goes up. | |
2 | N2+ + O2 → N2 + O2+ | 5.0e-11*xi**-0.8 | G18 | B92 used 5.1e-11*(T/300)**-0.8 S88 used 5.0e-11*(Tf/300)**-0.8 | |
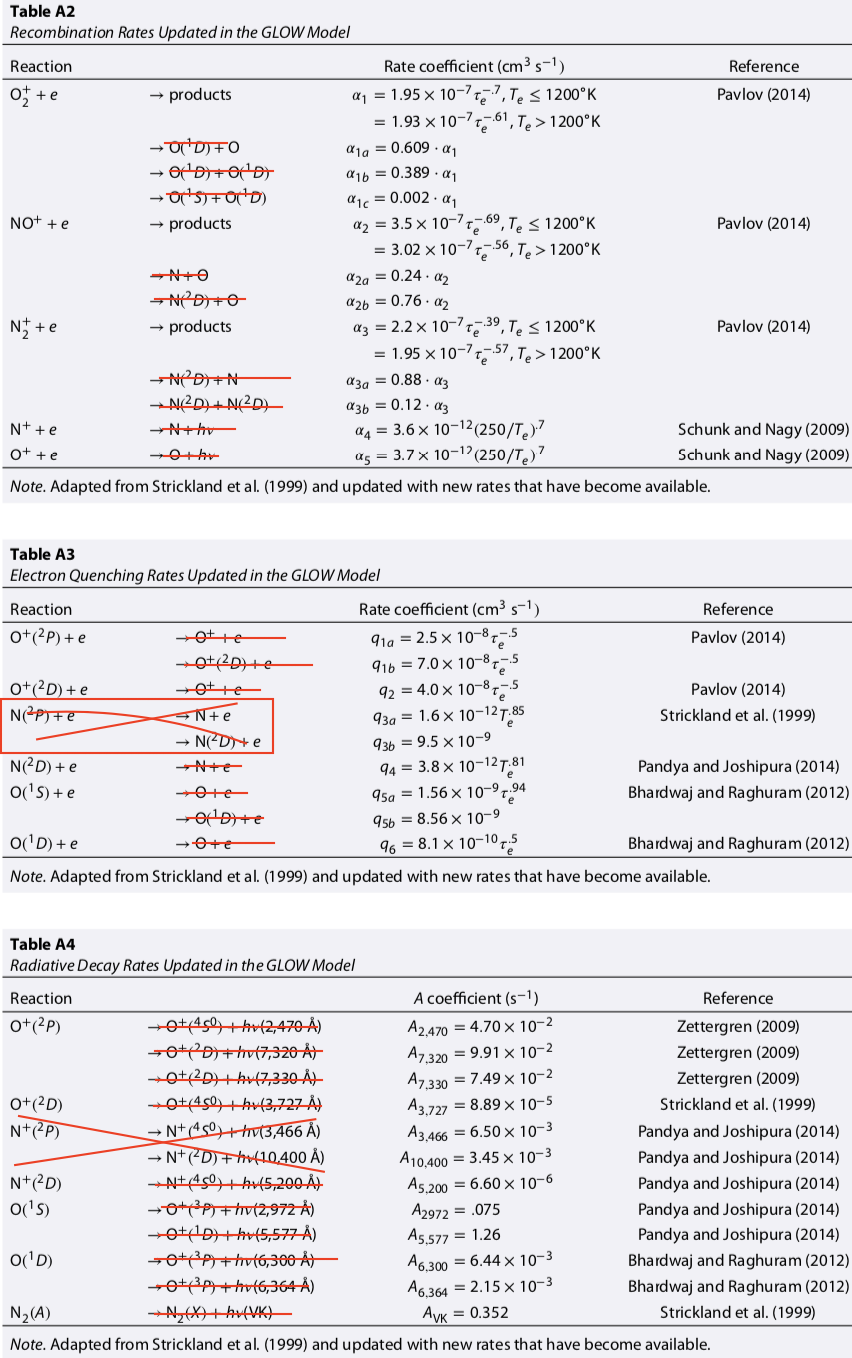
3 | N2+ + e → N(2D) + N | if Te<1200: 0.88*2.2e-7*xe**-0.39 if Te>=1200: 0.88*1.95e-7*xe**-0.57 | G18 | This has a dependence on the vibrational excitation of ions (Sheehan and St-Maurice 2004). They suggest using DR rate as a free parameter to infer ionic vibrational states, which are otherwise hard to measure. Valid up to 5000 K, vibrational states reduce by factor of 10 at high temps! B92 uses combined 1.8e-7*(Te/300)**-0.39 and doesn't give the yields S88 uses combined 3.5e-7*(Te/300)**-0.5 with 1.9 yield for N2D These two are discontinuous at Te=1200, but what are you gonna do? | |
72 | N2+ + e → N(2D) + N(2D) | if Te<1200: 0.12*2.2e-7*xe**-0.39 if Te>=1200: 0.12*1.95e-7*xe**-0.57 | G18 | Split the above into two reactions | |
4 | O+ + N2 → NO+ + N | if Tf<3725: 1.71676e-12 - 7.19934e-13*xf + 1.33276e-13*xf**2 - 9.28213e-15*xf**3 + 6.39557e-16*xf**4 if Tf>=3725: -1.52489e-11 + 7.67112e-13*xf + 1.19064e-13*xf**2 - 1.30858e-15*xf**3 + 4.67756e-18*xf**4 | SL98 | "For Teff values between 4000K and 6000K the rates may be up to 20% larger below the F-peak" So I could turn this knob to create more NO in STEVE S88 has a different rate that is only valid < 900 K This is important for explaining SAR arc and SAID ionospheric composition G18 uses a rate from a D-region review paper, so I ignored that The rate of this reaction increases with N2(j) [noted in Anderson 1992 and Pavlov 2011] Updated in SL98 which is valid up to Te=30,000 K This is not valid below 300 K, so take care in the lower thermsphere. This appears to be slightly discontinuous at Tf=3725, and I'm not sure if it's my typo, their typo, or what. | |
5 | O+ + O2 → O2+ + O | if Tf<4800: 2.78932e-11 - 6.92612e-12*xf + 8.67684e-13*xf**2 - 3.47251e-14*xf**3 + 5.07097e-16*xf**4 if Tf>=4800: -1.74046e-11 + 3.02328e-12*xf - 2.39214e-15*xf**2 - 4.02394e-17*xf**3 | SL98 | See notes above | |
6 | O+ + NO → NO+ + O | if Tf<3800: 6.40408e-13 - 1.33888e-13*xf + 7.65103e-14*xf**2 - 3.11509e-15*xf**3 + 6.62374e-17*xf**4 if Tf>=3800: -7.48312e-13 + 2.31502e-13*xf + 3.0716e-14*xf**2 - 2.65436e-16*xf**3 + 7.76665e-19*xf**4 | SL98 | Not in B92 nor in S88 I'm a little confused about this one. It's not really that temperature/energy dependent, I guess This could be an important loss process for NO at high temp, I suppose Updated in SL98 | |
9 | O2+ + NO → NO+ + O2 | 4.1e-10 | G18 | S88 and B92 have 4.4e-10 | |
67 | O2+ + N2 → NO+ + NO | 0 | S21 | G18 has 5e-16. Personal communication with Dave Siskind: "The value of 5E-16 was a mistake in reading the literature. It was an upper limit for that rate- meaning its never been detected to occur. That upper limit was published in the 1970's and Fred Rees presented it as the actual value in his 1989 text. There were papers in the 1980's which tried to observe the reaction and failed at the 2E-18 level. Probably not important for the F region since there isn't much O2+. But for the D region, it can screw you up if you include it. I've been on a crusade for the last 20 years to get people to eliminate this rate. My only published discussion is in Gumbel et al [2003], see top of page 5. And I'm sure no one has read this!" | |
38 | N2+ + NO → NO+ + N2 | 7.5e-9*Tn**-0.52 | G18 | Not in S88 nor B92, unless I missed it | |
10 | O2+ + N → NO+ + O(1S) | 0.15*1.65e-10 | G18 | Z09 | B92 had 1.2e-10 combined Not sure if this should apply to N(2D) as well G18 is from Zettergren (2009) which is from Witasse (1991) which is from Frederick et al. [1976] -- I haven't cross-checked all of these. Need to double check this one |
71 | O2+ + N → NO+ + O(1D) | 0.85*1.65e-10 | G18 | Z09 | Split above into two reactions. I think Witasse (1991) only has the branching to O(1S) not O(1D). Need to double check this one -- I am highly skeptical. For now I'll keep it, trusting Grubbs. But I don't understand where he got this from -- there may be some confusion with N+ + O2 --> NO+ + O(1D) |
11 | O2+ + e → O(1D) + O | if Te<1200: 0.609*1.95e-7*xe**-0.7 if Te>=1200: 0.609*1.93e-7*xe**-0.61 | G18 | SS04 |
Split into 3 reactions |
69 | O2+ + e → O(1D) + O(1D) | if Te<1200: 0.389*1.95e-7*xe**-0.7 if Te>=1200: 0.389*1.93e-7*xe**-0.61 | G18 | SS04 | Split into 3 reactions |
70 | O2+ + e → O(1D) + O(1S) | if Te<1200: 0.002*1.95e-7*xe**-0.7 if Te>=1200: 0.002*1.93e-7*xe**-0.61 | G18 | SS04 | Split into 3 reactions Solomon [1991] states that the yield (here only 0.002!) is highly dependent on the vibrational excitation of O2+. It is very low at v=0, grows rapidly, maximizing at v=2. Average yield may be 0.02-0.15. |
12 | N+ + O2 → O2+ + N | 1.93e-10 | G18 | Eqs 12, 42, 13, 43, and 14 are all the same reactants. S88 has them as one equation with yields roughly equal to what is used by B92, but S88 ignores 14, which is minor but maybe important for NO S88 and B92 have a 50% larger rate for this, probably because the difference branches to N(2D) | |
42 | N+ + O2 → O2+ + N(2D) | 8.25e-11 | G18 | B92 and S88 ignored the N2D yield and put it all on N4S | |
68 | O2+ + N(2D) → N+ + O2 | 2.5e-10 | G18 | Reverse of above reaction. Not in B92 nor S88 | |
13 | N+ + O2 → NO+ + O(1D) | 1.98e-10 | G18 | S88 and B92 had somewhat similar (but B92 had no yields of O(1D)) | |
43 | N+ + O2 → NO+ + O | 4.95e-11 | G18 | S88 and B92 had somewhat similar (but B92 had no yields of O(1D)) | |
14 | N+ + O2 → O+ + NO | 2.8e-11 | G18 | B92 had a 25% higher rate | |
44 | N+ + NO → NO+ + N | 5.73e-9 * Tn**-0.44 | G18 | P14 | Not in B88 nor B92, probably b/c it's from a D-region review paper and gets slower with temperature |
45 | N+ + NO → N2+ + O | 7.08e-10 * Tn**-0.44 | G18 | P14 | Not in B88 nor B92, probably b/c it's from a D-region review paper and gets slower with temperature |
15 | N+ + O → O+ + N | 2.2e-12 | G18 | S88 has this listed but no yields I can find, so assume ground N4S B92 (and S88?) have k=1e-12 | |
16 | NO+ + e → O + N(2D) | if Te<1200: 0.76*3.5e-7*xe**-0.69 if Te>=1200: 0.76*3.02e-7*xe**-0.56 | G18 | SS04 | SS04 has rate valid to 5000 K but note that vibrational ionic states can reduce rate by factor of 3. S88 has (combined) 2.3e-7(Te/300)**-0.5 from Mul and McGowen [1979] with fN2D=0.76 Barth uses (combined) 4.2e-7 * (Te/300)**0.85 from Torr et al (1976b) with fN2D=0.75 I branched it into two reactions |
54 | NO+ + e → O + N | if Te<1200: 0.24*3.5e-7*xe**-0.69 if Te>=1200: 0.24*3.02e-7*xe**-0.56 | G18 | SS04 | I branched reaction 16 into two reactions |
17 | N + O2 → NO + O | 1.5e-11*exp(-3600/Tn) | B09 | Note that N(4S) also destroys NO G18 has 1.5e-11*exp(-3573/Tn) from Strickland et al (1999) B92 has 4.4e-12*exp(-3220/T) B09 has 1.5e-11*exp(-3600/T) P05 has 1.1e-14 * T * np.exp(-3150/T) (increased NO in my toy model by 33%, but maybe not applicable in our temperature regime) SN09 has 4.4e-12*exp(-3220/Tn) Whatever I use it should be consistent with loss rate, 21. What if this rate goes way up with O2*? Aladjev and Kirillov have a discussion of this. But since [N2] > [O2] and [O] > [N], I'll focus on O+N2(v) for NO production. | |
76 | N + O → NO | 3.33e-16 * Tn**-0.5 * (1 - 0.567*Tn**-0.5) | G18 | This reaction produces IR radiation which is ignored here | |
18 | N(2D) + O2 → NO + O | 0.9*6.2e-12*xn | G18 | D03 |
|
49 | N(2D) + O2 → NO + O(1D) | 0.1*6.2e-12*xn | G18 | D03 | I created two reactions out of the reaction above, so the same notes above apply here. This is probably such a small source of O(1D) which will never radiate anyway. (But some sources say it is the main source of O(1D) in the airglow and aurora?!) |
50 | N(2D) + N2 → N + N2 | 1e-13 * exp(-510/Tn) | G18 | Not in S88 nor B92, probably b/c it's negligible | |
19 | N(2D) + e → N + e | 3.8e-12 * Te**0.81 | G18 | B92 uses 6e-10 * (Te/300)**0.5 but from the same ultimate source as S88?? S88 uses 5.5e-10 * (Te/300)**0.5 from the same source as B92?? | |
20 | N(2D) + O → N + O | 1.26e-12 | G18 | S88 combined rate is 1.8e-12 from Davenport et al (1976) B92 combined rate is 1e-12 from Fesen et al (1989) & Siskind (1988) | |
51 | N(2D) + O → N + O(1D) | 1.4e-13 | G18 | I branched reaction 20 into 3 separate branches. B92 has this one at 0.1 | |
52 | N(2D) + O → NO+ + e | 2.5e-18 * Tn**0.5 * (2205+Tn)*exp(-4410/Tn) | G18 | I branched reaction 20 into 3 separate branches. Neither B92 nor S88 has this one, probably negligible | |
53 | N(2D) + O+ → N+ + O | 1.3e-10 | G18 | Not in S88 nor B92, probably b/c it's negligible | |
21 | N + NO → N2 + O | 1.6e-10*exp(-460/Tn) | B09 |
| |
22 | N(2D) + NO → N2 + O | 7e-11 | B92 | Maybe this produces N2* like the above? | |
38 | N(2D) + NO → N + NO | 6.7e-11 | G18 | (Not colored gray because NO is neither created nor destroyed) S88 has 7e-11 | |
23 | O + e → O(1D) + e | 0.15 * Te**0.5 * (8537 + Te)/(34191 + Te)**3 * exp(-22756/Te) | MC91 | This is the SAR arc mechanism and should be checked.
| |
24 | O(1D) + N2 → O + N2 | 1.8e-11*exp(107/Tn) | G18 | TS14 | S88 has 2e-11*exp(107.8/Tn) which is similar Kalogerakis et al (2009) has a discussion of a new 2006 NASA/JPL recommended rate that is higher than these two values. See plot below. This will make a difference in red line rate and profile! |
25 | O(1D) + O2 → O + O2 | 3.2e-11*exp(67/Tn) | G18 | TS14 | S88 has 2.9e-11*exp(67.5/Tn) which is similar |
59 | O(1D) + NO → O + NO | 1.5e-10 | G18 | S99 | Not colored since NO is just a quencher Not usually considered for red line, but might help understand red line in STEVE |
26 | O(1D) + e → O + e | 8.1e-10 * xe**0.5 | G18 | S88 has the exact same rate | |
37 | O(1D) + O → O + O | 2.5e-11 | G18 | K09 |
|
27 | O(1S) + O → O + O | 2e-14 | G18 | Presumably it doesn't matter what state O is in? G18 and S88 agree | |
55 | O(1S) + O2 → O + O2 | 1.6e-12 * exp( -(6750-0.0151*Tn**2)/(8.314*Tn)) | G18 | ||
74 | O(1S) + e → O + e | 1.56e-9 * xe**0.94 | G18 | ||
75 | O(1S) + e → O(1D) + e | 8.56e-9 | G18 | ||
56 | O(1S) + O2 → O(1D) + O2 | 7.2e-13 * exp( -(6750-0.0151*Tn**2)/(8.314*Tn)) | G18 | ||
57 | O(1S) + NO → O(1D) + NO | 5.12e-11 | G18 | Not colored since NO is just a quencher Could this process explain the lack of green seen in STEVE? One would expect green emission but the elevated NO quenches it significantly | |
58 | O(1S) + NO → O + NO | 2.88e-11 | G18 | Not colored since NO is just a quencher | |
72 | N+ + e → N | 3.6e-12*(250/Te)**0.7 | G18 | This emits, probably in the FUV somewhere like 1356 does for RR of O+, which is ignored here | |
73 | O+ + e → O | 3.7e-12*(250/Te)**0.7 | G18 | This emits at 1356 | |
101 | O(1D) → O + hv6300A | 8.59e-3 | G18 | In this model 6300 and 6364 are combined into a single emission | |
102 | O(1S) → O(1D) + hv5577A | 1.26 | G18 | ||
103 | O(1S) → O + hv2972A | 7.5e-2 | G18 | ||
104 | N(2D) → N + hv5200A | 6.6e-6 | G18 | B92 has 1.07e-5 | |
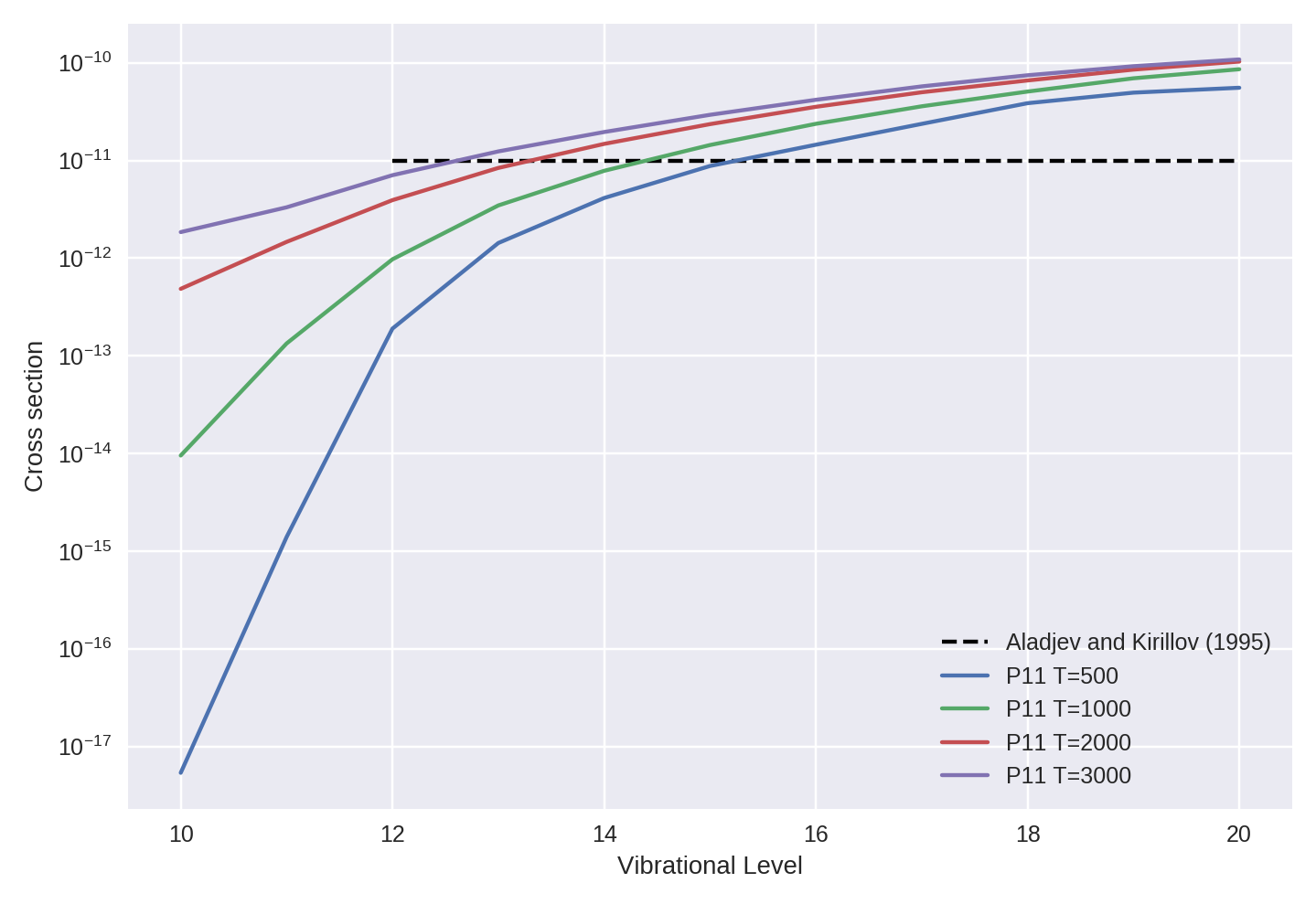
109 | N2(v>11) + O → NO + N | 1e-11 | P11 | See P11 p164 |
|
112 | N2(v>4) + O(1D) → NO + N | This was speculated in Liang et al (2020). I've never seen a reaction rate for it, but I think it could be very important for STEVE if there's enough O(1D). It could even be a missing sink of O(1D). It makes sense energetically, but I'm not sure about the rate. It significantly lowers the vibrational level threshold and opens us up to many sources of vibrational excitation. |
41 | O + N2 → NO + N | Zipf et al 1970 | Suggested by Zipf et al (1970) but I haven't seen it anywhere else. It requires the O to be moving fast to achieve activation energy, which is going to require a whole bunch of kinetic theory since it's a neutral.... Wait - this is the same reaction as the one below. I guess it actually happens if the N2 is vibrationally excited. | ||
111 | N + O2(v) → NO + O | Aladjev and Kirillov (1995) mention this one. This is perhaps a special case of the above ground-state reaction, which is the primary mechanism of NO production.... or something. They have a v-dependent rate coefficient which I would have to think about the parameters for. | |||
34 | O2* + O → O2 + 0.1 O(1S) + 0.9 O | 2.1e-11*exp(-1136/Tn) | S88 | What is O2*? S88 says 0.1 branching to O1S. I guess the rest goes to O3P? | |
35 | O2* + N2 → O2 + N2 | 1e-13 | S88 | Can this excite N2(A)? TODO: Double check if this should be N2* | |
109 | NO + O → NO2 + hvcont | 1e-20 | H12 | This doesn't participate in the chemistry because it's so slow and because the NO2 gets recycled back into NO quickly. It will just be used to compute the brightness. Hedin et al (2012) uses 1e-20 valid for 540 nm. Sharp (1984) suggests temp dependence that hasn't been validated: 9e-20 * exp(-1060/(RT)) or in Hedin's translation: 1e-20*exp(530 * (1/296 - 1/T)). Most studies show results with and without T dependence Note that this gives ph/nm, technically only valid at 540 nm. The 3-body reaction path, while interesting, is omitted here. I should maybe include it to determine if the full altitude profile makes sense. Quenching should for sure be included if so. I wonder if NO* could crank up this rate? | |
110 | N(2D) + O2 → NO2 + hvcont | 6.2e-12*xn * B??? |
| ||
108 | N2(A) → N2 + hvVK | 0.352 | G18 | ||
105 | O+(2P) → O+ + hv2470A | 4.7e-2 | G18 | ||
106 | O+(2P) → O+(2D) + hv7320A | 1.74e-1 | G18 | G18 separates 7320 and 7330 emissions | |
107 | O+(2D) → O+ + hv3727A | 8.89e-5 | G18 | ||
36 | N2(A) + O → N2 + O(1S) | 1e-11 | G18 | Z09 | According to Henriksen and Egeland, 1988, this is the dominant source of green line in aurora. S88 says 0.75 branching to O(1S). I guess the rest goes to O3P? S88 uses 3.11e-11 combined (ave v=1,2) C06 uses 2.5e-11(Tn/298)**0.55 and doesn't mention branching, seeming to imply 100% O(1S). I am worried about that temperature dependence applying at high T, so I'm going to use G18 from Z09, which has 0.36 branching to O(1S) Split into two reactions |
60 | N2(A) + O → N2 + O | 1.8e-11 | G18 | Z09 | Split into two from the reaction above |
40 | N2(A) + O → NO + N(2D) | 2e-11 | C06 | (C07) This dominates over the similar N2(v) reaction below in aurora, but N2(v) is more important at alt > 160 km | |
37 | N2(A) + O2 → N2 + O2 | 4e-12 | G18 | S99 | S88 has 4.1e-12 |
61 | N2(A) + NO → N2 + NO | 8.9e-11 | G18 | S99 | |
62 | N2(A) + N → N2 + N(2D) | 4e-12 | G18 | S99 | I'm not including the branch to N(2P) |
7 | O+(2D) + N2 → N2+ + O | 1.5e-10 * xn**-0.5 | G18 | S88 and B92 have 8e-10 | |
46 | O+(2D) + N2 → O+ + N2 | 8e-10 | G18 | S88 and B92 ignored this quenching reaction | |
8 | O+(2D) + O2 → O2+ + O | 1e-10 * xn**-0.5 | G18 | S88 has 7e-10 from Johnsen and Bionde [1980] B92 has 1e-9 is from Torr and Torr [1982] | |
47 | O+(2D) + NO → NO+ + O | 1.2e-9 | G18 | Not in S88 nor B92. Seems like a high reaction rate, but low population I guess | |
64 | O+(2D) + N → N+ + O | 7.5e-11 | G18 | Not in S88 nor B92 | |
28 | O+(2D) + e → O+ + e | 4e-8*xe**-0.5 | G18 | S88 has 6.6e-8 * (Te/300)**-0.5 | |
29 | O+(2D) + O → O+ + O(1D) | 2.5e-12 | S88/G18 | G18 has 5e-12 total and doesn't have branch to O(1D) S88 has 1e-11, and I'm keeping his 0.5 branch to O(1D) This is probably a negligible reaction, but if it's important I should double check the sources. | |
48 | O+(2D) + O → O+ + O | 2.5e-12 | G18 | G18 doesn't have branch to O(1D) S88 has 1e-11, and I'm keeping his 0.5 branch to O(1D) | |
30 | O+(2P) + N2 → O + N2+ | 2e-10 * xn**-0.5 | G18 | S88 has 4.8e-10 | |
63 | O+(2P) + N2 → N+ + NO | 1e-10 | G18 | Not in S88 nor B92 | |
31 | O+(2P) + O2 → O + O2+ | 3.1e-10 * xn**-0.5 | G18 | S88 has 4.8e-10 | |
65 | O+(2P) + N → N+ + O | 1e-10 | G18 | Not in S88 nor B92 | |
66 | O+(2P) + NO → NO+ + O | 2.9e-8 | G18 | Not in S88 nor B92 | |
32 | O+(2P) + e → O+ + e | 2.5e-8 * xe**-0.5 | G18 | Seems irrelevant for high Te situations predicting 732 emission at first glance. S88 has 1.7e-7*(Te/300)**-0.5 (combined) | |
73 | O+(2P) + e → O+(2D) + e | 7.0e-8 * xe**-0.5 | G18 | Split into two reactions | |
33 | O+(2P) + O → O+ + O | 5.2e-11 | G18 | S88 has 5e-11 |